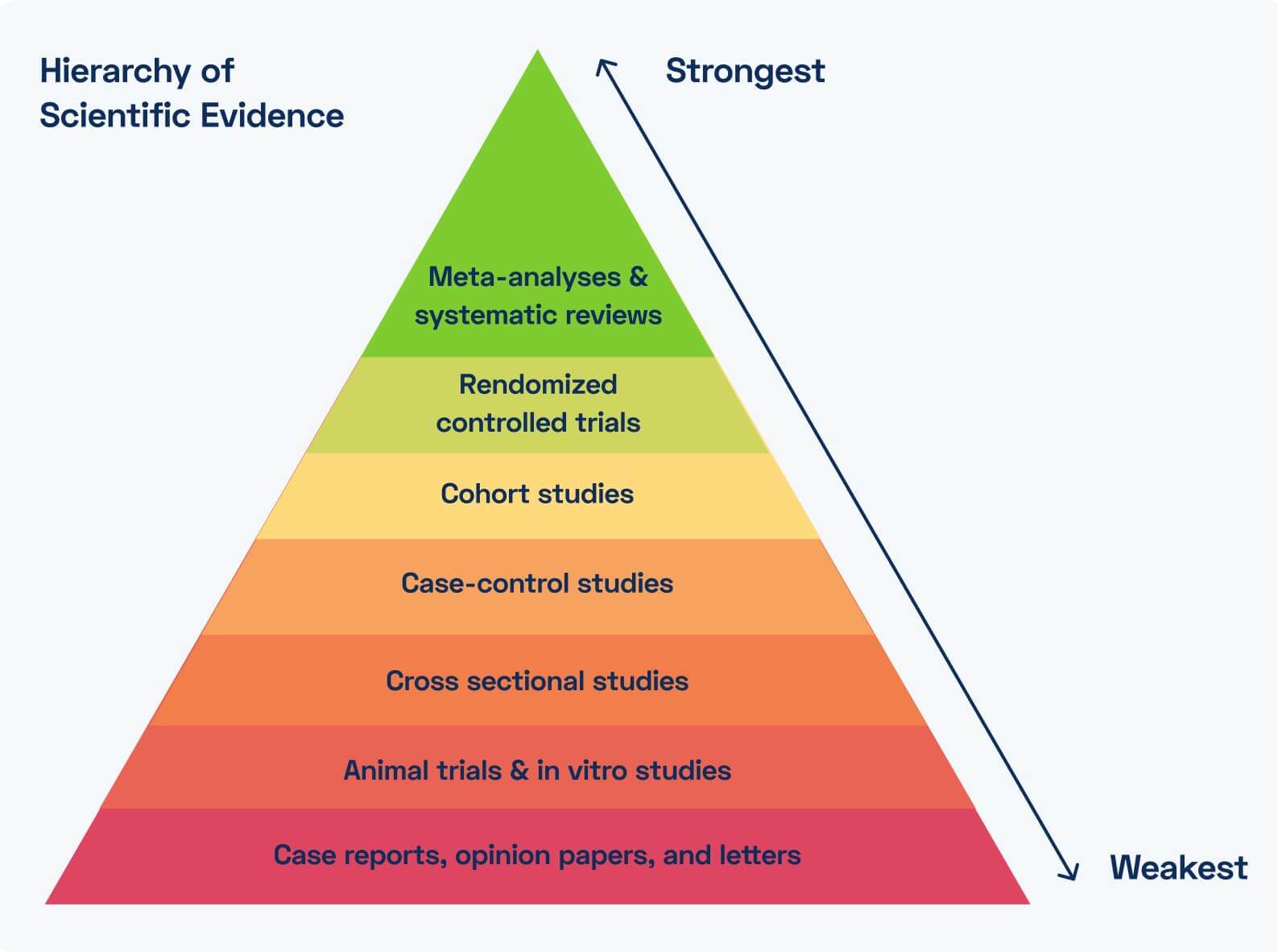
The Value of Evidence & Clinical Research in MSK Digital Therapeutics
How clinical evidence can help providers and employers choose the best digital MSK therapies for their populations
6 min read

Further Reading
-
 At Kaia, we know a thing or two about jump-starting motivation. Read on and we’ll show you how with our top 7 tips for getting—and staying—motivated to exercise daily.8 min read
At Kaia, we know a thing or two about jump-starting motivation. Read on and we’ll show you how with our top 7 tips for getting—and staying—motivated to exercise daily.8 min read -

Kaia Health’s COPD Pulmonary Rehabilitation App to Launch in Europe
Historic agreement with Chiesi Group forges innovative digital therapeutics-pharmaceutical partnership on at-home respiratory care therapy solution, builds foundation for U.S. market3 min read -

Kaia Health SMART Coaching Model Drives Digital MSK User Experience, Engagement, and Outcomes
Highly individualized coaching experience and exercise goals maximize therapeutic effectiveness while reducing MSK medical spend.4 min read